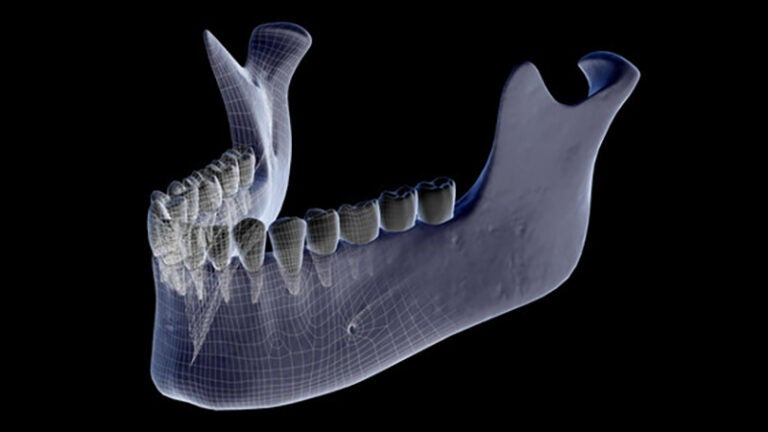
Osteonecrosis of the jaw is a rare, incurable side effect of some drug treatments. (Photo/Science Source)
New research could prevent jaw damage in patients being treated for cancer or osteoporosis
The new technique could play a big part in eliminating osteonecrosis of the jaw, a currently incurable rare side effect of certain drugs.
USC researchers and collaborators report a breakthrough to prevent damage to the jaw, a side effect suffered by some people undergoing treatment for cancer or osteoporosis.
The newly published research is an important step toward a cure for osteonecrosis of the jaw, a rare side effect caused by drugs commonly used to combat bone loss. It causes severe and persistent inflammation, leading to loss of bone from the jaw, and has no effective prevention or cure. The risk, though small, deters people from taking drugs needed to fight bone cancer or prevent fractures due to loss of bone density.
USC scientist Charles McKenna said the successful animal experiment, conducted by researchers at USC and UCLA, raises hope that physicians could adapt the new method to treat the condition in people.
“This is a condition that has been excruciatingly painful and difficult to treat for more than a decade,” said McKenna, a professor of chemistry in the USC Dornsife College of Letters, Arts and Sciences and adjunct professor of pharmacology and pharmaceutical sciences in the USC School of Pharmacy. “We think our new approach may provide hope for the future.”
The published findings appear in Bone. The authors are affiliated with the USC Center for Drug Discovery and Development at the USC Michelson Center for Convergent Bioscience, the UCLA School of Dentistry and BioVinc LLC, a Pasadena-based startup biotech company.
Jaw damage: What causes it
For years, physicians have prescribed a class of drugs called bisphosphonates (BPs) to treat metastatic bone cancer patients and to maintain bone density in osteoporosis patients. BPs include a range of compounds that share a remarkable ability to stick to bone like Velcro.
But when used in high doses in the cancer clinic, BP drugs sometimes have a terrible side effect that causes necrosis in the jaw. The problem often occurs after a tooth is removed; the gap doesn’t heal and the jaw begins to deteriorate.
Although the condition is very rare at the lower BP doses used to combat osteoporosis, many patients are avoiding the drugs altogether for fear of the side effects. The National Osteoporosis Foundation estimates incidence of osteonecrosis of the jaw due to BPs to be between 1 in 10,000 and 1 in 100,000 people annually. The risk is higher — about 3 percent of patients — at the BP dose used to treat cancer, McKenna said.
Nonetheless, more and more osteoporosis patients are willing to take their chances with the disease rather than risk the side effects. Surveys have shown the recent trend in reduced hip fractures among postmenopausal women may be reversing due to BP drug aversion.
“The fear factor of this condition has led to severe underuse of bisphosphonates for osteoporosis, so much so that we’re seeing a rise in hip fractures in elderly people, aversion to bisphosphonates in oncology clinics and liability concerns in the dental office,” McKenna said.
Jaw damage: How to prevent it
To solve the problem, McKenna devised an elegant solution. The research team used a different inactive BP compound that could be used locally in the mouth to push the BP drug from the jawbone while leaving undisturbed the useful drug in the rest of the skeleton.
Said McKenna: “Think of it as a way to fight fire with fire.”
The scientists involved in the study used mice to test different BPs attached to fluorescent dyes. One color label coded the BP zoledronate, which is administered systemically to treat osteoporosis and cancer, while a different color labeled “rescue BP” coded a BP compound with similar bone affinity but no biological activity. The researchers discovered that rescue BP injected into the jaw removed most of the BP drug that caused the jawbone tissue damage, clearing the way for the animal’s natural healing process to repair the extraction site.
The new technique isn’t yet ready for clinical use in humans. McKenna said BioVinc, which provided funding for the study via a National Institutes of Health small business research grant, will be responsible for advancing the treatment to commercial clinical use. Several of the authors of the study disclose a financial interest in BioVinc, a company specializing in “bone-targeted therapeutics and diagnostics.” McKenna is the company’s academic founder.
USC’s McKenna is a corresponding author of the study. Other authors include Akishige Hokugo, Keiichi Kanayama, Shuting Sun, Kenzo Morinaga, QingQing Wu, Hodaka Sasaki, Hiroko Okawa, Courtney Evans and Ichiro Nishimura of the UCLA School of Dentistry and Frank H. Ebetino, Mark W. Lundy and Keivan Sadrerafi of BioVinc.
The study was supported by NIH/NIDCR grants (1R43DE025524, 2R44DE025524, R01DE022552, R21DE023410), an NIH/NCRR grant (C06RR014529) and by USC Dornsife. Technical support was provided by the UCLA Translational Pathology Core Laboratory and Matthew J. Schibler of UCLA.