Keck Medicine of USC offers FDA-approved sleep apnea implant
Device provides relief for patients plagued with chronic snoring
Keck Medicine of USC is the first medical center in Los Angeles to offer a unique implantable treatment for sleep apnea, a chronic disorder that affects more than 18 million sleep-deprived Americans.
Keck Medical Center of USC patients who qualify as candidates now may consider the Inspire Upper Airway Stimulation system, approved by the Food and Drug Administration on April 30 for moderate to severe obstructive sleep apnea.
Often characterized by chronic snoring, sleep apnea is a common disorder that occurs when breathing is repeatedly interrupted during sleep, leading to daytime sleepiness as well as possible health problems. The tongue is a major contributor to sleep apnea for many patients. The Upper Airway Stimulation system monitors a patient’s breathing patterns and sends an electrical pulse to the nerve that controls the tongue to keep the airway open.
“The implant truly is a breakthrough for people who are unable to tolerate the standard treatment for sleep apnea,” said Keck Medicine of USC surgeon Eric Kezirian, professor of clinical otolaryngology at the Keck School of Medicine of USC.
Kezirian, an international leader in the surgical evaluation and treatment of snoring and sleep apnea recruited to USC from the University of California, San Francisco, has conducted key research studies regarding nerve stimulation for sleep apnea. Keck Medical Center of USC this year ranked among the top 25 percent of U.S. hospitals that provide specialized ear, nose and throat care.
Current treatments
The standard treatment for sleep apnea includes use of a continuous positive airway pressure device (CPAP) — a mask that fits over the nose or mouth and blows air into the airway to help keep it open during sleep. Approximately half of apnea patients, however, are unable to sleep comfortably while wearing positive airway pressure device and should consider other options, Kezirian said.
Other treatments include lifestyle changes such as weight loss, surgery and oral appliances. Left untreated, the combination of disturbed sleep and oxygen starvation may lead to escalating health risks such as hypertension, heart disease and mood and memory problems, according to the National Sleep Foundation.
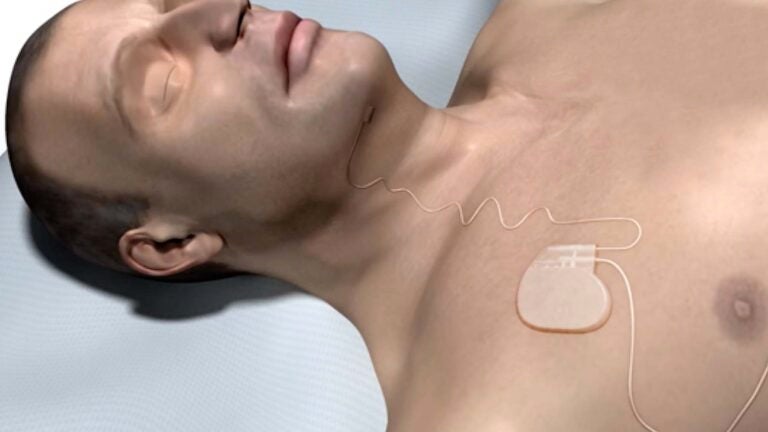
All components of the Upper Airway Stimulation system are placed inside the body during a minimally invasive two-hour surgical procedure. The implant consists of a small generator that is positioned in the upper chest like a pacemaker and electrical leads that enable the generator to stimulate the nerve that controls the tongue to keep the throat open while the patient is breathing during sleep.
People who qualify for Inspire therapy have been diagnosed with obstructive sleep apnea, either failed or not tolerated CPAP treatment, have a body-mass index of 32 kg/m2 or less and do not have any other active implantable devices, such as a pacemaker.
FDA approval was based on a clinical trial conducted at 22 medical centers across the United States and Europe. The study, published in The New England Journal of Medicine, found major improvements in sleep apnea and snoring.
The typical patient implanted with the device experienced a 68 percent reduction in sleep apnea and significant improvements in quality of life and daytime functioning. Moreover, 85 percent of bed partners reported either no snoring or soft snoring for their partners using the device.
For more information, or to make an appointment, go to ent.keckmedicine.org or call (800) USC-CARE.



