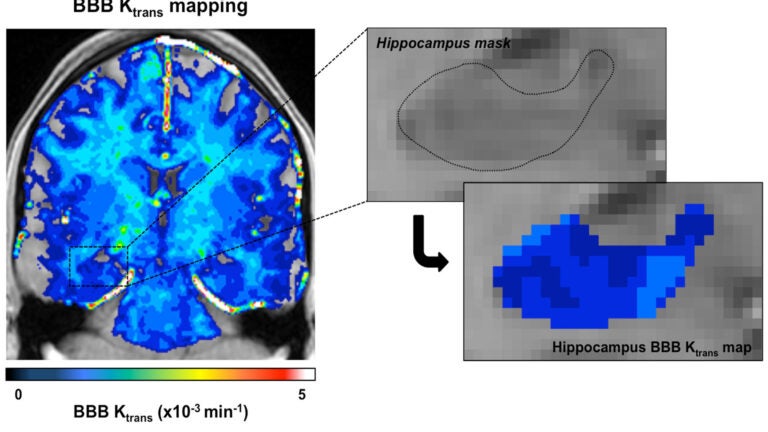
USC researchers used enhanced brain images to measure the blood-brain barrier’s permeability, finding that the barrier becomes leaky with age. (Photo/Zlokovic Lab/Keck Medicine of USC).
Scans detect aging brain issues linked to dementia
Advanced image analysis suggests a breakdown in the brain’s memory and learning center can be detected before cognitive loss begins
USC neuroscientists may have unlocked another puzzle to preventing risks that can lead to Alzheimer’s disease.
Researchers at Keck Medicine of USC used high-resolution imaging of the living human brain to show for the first time that the brain’s protective blood barrier becomes leaky with age, starting at the hippocampus, a critical learning and memory center that is damaged by Alzheimer’s disease.
The study indicates it may be possible to use brain scans to detect changes in blood vessels in the hippocampus before they cause irreversible damage leading to dementia in neurological disorders characterized by progressive loss of memory, cognition and learning.
A ‘significant step’
The findings would have broad implications on conditions that will affect 16 million Americans over age 65 by 2050, according to the latest figures from the Alzheimer’s Association. The research appears in the Jan. 21 edition of the peer-reviewed scientific journal Neuron.
This is a significant step in understanding how the vascular system affects the health of our brains.
Berislav Zlokovic
“This is a significant step in understanding how the vascular system affects the health of our brains,” said Berislav Zlokovic, director of the Zilkha Neurogenetic Institute at the Keck School of Medicine of USC, holder of the Mary Hayley and Selim Zilkha Chair for Alzheimer’s Disease Research and the study’s principal investigator. “To prevent dementias including Alzheimer’s, we may need to come up with ways to reseal the blood-brain barrier and prevent the brain from being flooded with toxic chemicals in the blood. Pericytes are the gatekeepers of the blood-brain barrier and may be an important target for prevention of dementia.”
Alzheimer’s is the most common type of dementia, a general term for loss of memory and other mental abilities. According to the Alzheimer’s Association, roughly 5.2 million people of all ages in the United States have Alzheimer’s disease, an irreversible, progressive brain disease that causes problems with memory, thinking and behavior. Postmortem studies of brains with Alzheimer’s show damage to the blood-brain barrier, a cellular layer that regulates entry of blood and pathogens into the brain. The reasons why and when this damage occurs, however, remain unclear.
Brain images
In the Neuron study, Zlokovic’s research team examined contrast-enhanced brain images from 64 human subjects of various ages and found that early vascular leakage in the normally aging human brain occurs in the hippocampus, which normally shows the highest barrier properties compared to other brain regions. The blood-brain barrier also showed more damage in the hippocampal area among people with dementia than those without dementia, when controlling for age.
To validate the research method, the USC team examined brain scans of young people with multiple sclerosis without cognitive impairment, finding no difference in barrier integrity in the hippocampus between those of the same age with and without the disease.
The researchers also looked at the subjects’ cerebrospinal fluid (CSF), which flows through the brain and spinal cord. Individuals who showed signs of mild dementia had 30 percent more albumin, a blood protein, in their CSF than age-matched controls, further indicating a leaky blood-brain barrier.
The CSF of individuals with dementia also showed a 115 percent increase of a protein related to pericyte injury. Pericytes are cells that surround blood vessels and help maintain the blood brain barrier; previous research has linked pericytes to dementia and aging.
Study participants were recruited through the USC Alzheimer’s Disease Research Center and Huntington Medical Research Institutes. Other USC co-authors include Axel Montagne, Melanie Sweeney, Matthew Halliday, Abhay Sagare, Zhen Zhao, Arthur Toga, Collin Liu, Lilyana Amezcua, Helena Chui and Meng Law.
The study was supported by various National Institutes of Health agencies (grants R37NS34467, R37AG23084, R01AG039452, R21EB013456, UL1TR000130, P50AG05142, 7P41EB015922, EB000993), the Zilkha Senior Scholar program and L. K. Whittier Foundation.